I recently posted statistics on number of adverse reactions and deaths associated with the use of isoxazoline drugs reported to the EMA (European Medicines Agency), which drew a large reaction on social media. Some people were maddened by the number of animals harmed by these chemicals, while others were irate because I did not post a "study" showing the numbers. I explained repeatedly that these numbers were valid, as they were requested from, and sent by, the EMA. The EMA is not allowed to publish these numbers, but we are allowed to request the number of reports and can do as we please with the numbers.
In the United States, the FDA is the equivalent of the EMA in Europe. While the FDA also does not publish numbers of adverse reactions and deaths associated with pet food or drugs, consumers can request those numbers through the Freedom of Information Act. Sometimes it takes many months to receive the reports and they come with a lot of information blacked out.
But I do happen to have in my possession some of the numbers of reported adverse reactions to the FDA and the EMA for the isoxazoline drugs. The most interesting and obvious difference is the percentage of deaths and adverse reactions between the two agencies.
FDA 1/13 to 9/17:
32,374 reports of adverse reactions or death:
801 deaths (2.5%)
1728 seizure reports (5.3%)
EMA 1/13 to 1/19:
39,148 reports of adverse reactions or death:
5556 deaths (14.2%)
6272 seizure reports (16%)
Why were there 5.7 times more deaths reported in Europe than the United States for the same drugs? Why were there 3 times more animals affected with seizures reported in Europe than the United States? Does the FDA have a different way of "analyzing data"?
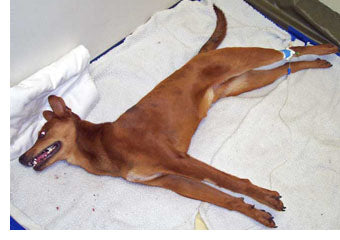
I got a clue to how the reporting works when my office received a phone call the other day from a pharmaceutical company that manufactures one of the isoxazoline drugs. A client had reported that her pet had seizures related to the drug. The dog also had vaccinations and was given a monthly heartworm preventative around the same time. The manufacturer's response to us? The seizures could not possibly be related to their drug; they must be related to the heartworm preventative or vaccines.
Did this report even make it to the FDA? Or did the company divert this report as "not relevant"? We will never know.
If your pet has a suspected adverse reaction to ANY chemical or vaccine, do not leave it up to your veterinarian to report to the FDA (most won't take the time because they are incredibly busy and many don't know where to make the report). Make sure you report to the drug or vaccine manufacturer, as well as the FDA. This dog had seizures I suspect were related to the isoxazoline drug (which is labeled as potentially causing seizures). However, in fairness, the seizures should have been reported to the manufacturers of the heartworm preventative and every one of the vaccines the pet received within the months prior to the seizures starting.
Until we make sure that all adverse events are reported, we will never make changes to the use and labeling of these drugs and vaccines. It is estimated that only 1 to 10% of adverse reactions are reported. As reported in this paper, only a small percentage of people are even aware that they can report an adverse reaction. If all were reported, the numbers would be very sad to digest.